|
Mind
Blindness and the Brain in Autism By Uta Frith In
this review, I will discuss a productive and successful, though still
controversial, theory of autism. This theory attempts to explain the social
and communication failure that is the very core of autistic disorder. The
cognitive cause for this failure is assumed to be “mind
blindness.” This concept presupposes that normal individuals have the
capacity to “mind read,” that is, to attribute mental states to
self and other. This is referred to as the “theory of mind” or
“mentalizing.” The theory assumes that this capacity, far from
being the product of complex logical inference, rests on a dedicated
neurocognitive mechanism. I will review the evidence that this mechanism is
impaired in both severe and mild forms of autism. Its putative neural basis
can give clues to the underlying brain abnormalities in autism.
The Autism Spectrum It
is now widely agreed that autism is a neurodevelopmental disorder (for
reviews see Bailey et
al. 1996; Happé
and Frith 1996 and Lord et al.
2000). Autism persists throughout life. It varies in degree of
severity and can occur at all levels of ability, so that it is now generally
assumed that there is a spectrum of autistic disorders. Asperger syndrome, a
milder variant, and currently distinguished from other forms of autism by the
lack of linguistic or cognitive delay, is often not diagnosed until late
childhood or even adulthood. The diagnosis of autistic disorder is based on
behavioral criteria set out in diagnostic handbooks such as the ICD-10 (World
Health Organization, 1992) and DSM-IV (American
Psychiatric Association, 2000). Autism was first identified and
labeled by Kanner
(1943) and Asperger
(1944). The
causes of autism are largely genetic (see Maestrini
et al., 2000, for a review of susceptibility genes). There is no
known medical treatment, but well-structured behavioral treatments have
beneficial effects, and high levels of compensatory learning can occur. The
prevalence of autism spectrum disorders is now estimated at between 0.3% and
0.7%. The increase of diagnosed cases in recent years can be accounted for by
increased awareness of the disorder in all its variants and the use of wider
diagnostic criteria (Fombonne,
1999). The male to female ratio is approximately 3 to 1, becoming
more extreme with higher levels of ability. Individuals
with autistic spectrum disorder have striking limitations in social
relatedness and in the ability to communicate verbally and nonverbally. They
are often aloof in childhood and remain egocentric even after having learned
the basic rules of social interaction. They may have no speech, or very
delayed speech, and even those who become verbally fluent still have problems
in comprehension. Individuals with autism also have other characteristic
features, such as restricted interests, motor stereotypes, and obsessive
tendencies. They can have excellent rote memory and may possess savant
skills. Autism
is a disorder that affects many cognitive functions; however, it does not
imply a global information processing deficiency (Scheuffgen
et al., 2000). While the hallmark of the disorder is a failure of
social communication, this does not imply a global lack of social ability.
Rather, autism appears to be caused by one or more specific, i.e.,
circumscribed, cognitive deficits. At the same time, such modular deficits
would have developmental repercussions on general adaptive functioning (Frith and
Happé, 1998). This is in line with current ideas about
innate domain-specific mechanisms with a circumscribed basis in the brain
(for a discussion of current theories see Black, 1998).
Arguably the most relevant of these deficits in the origin of autism is a
subtle but devastating deficit in human social insight, on which this review
will focus. This can be referred to as the mind blindness hypothesis (e.g., Baron-Cohen,
1995). Mind Reading and Mind Blindness Individuals
with autistic disorder have occasionally commented on what they perceive as
an unfathomable yet ubiquitous ability of other people to “mind
read” during ordinary social interactions. Normal people indeed behave
as if they have an implicit theory of mind, and this allows them to explain
and predict others' behavior in terms of their presumed thoughts and
feelings. To give an example: you might observe me in my office bent over a
filing cabinet drawer pulling out and putting back folders. You would make
sense of this behavior by mentalizing, that is, automatically recognizing
that I am looking for a paper that I believe is in one of the folders
and that I wish to retrieve. You would think this even if you knew
that the paper was not there. To explain my behavior, it is immaterial
whether the missing file is in the cabinet or really somewhere else. Suppose
that you say to me “Try Debbie's desk,” and I respond with
“I might have known.” Without mentalizing, this everyday exchange
would seem like complete non sequiturs. Further, without mentalizing, you
might come up with an outlandish interpretation of what I was
doing—perhaps practicing back bending and finger moving? The important
point of the example is that for an instantaneous interpretation of ordinary
behavior, we automatically take account of the mental state of people, their
desires, and their beliefs. The Cognitive Basis of Mind Reading Leslie
(1987) proposed that the ability to represent mental states is based
on a dedicated cognitive mechanism. This mechanism includes a
“decoupler” and an “expression raiser” and transforms
primary representations (impressions of the physical world) into secondary
representations. These are “decoupled” from reality and raised
into expressions “in quotes.” They can thus be attached to an
agent's intentional stance; for example, agent A believes, desires,
etc., that “x is the case.” Mentalizing can thus be
conceived of as representing an agent's propositional attitude to states in
the world, thus keeping apart someone's attitude to states in the world and
actual states in the world. This is why children are not confused when their
mother holds a banana to her face and pretends it is a telephone. According
to Leslie
(1987), the first florid manifestation of the ability to mentalize
is seen in the young child's enjoyment of pretence, from around 18 months.
Here the child acts as if realizing that when mother is using a banana as a
telephone, she is taking a propositional attitude to a particular object,
which does not interfere with the child's learning about real telephones and
real bananas. The implications of this proposal are radical: a neural system
is required that supports the processing of specific information in relation
to agents and is not tied to a particular modality. If there is such a system
in the normal case, then we can envisage this system being dysfunctional from
birth, resulting in a difficulty with the intentional stance. This difficulty
would result in mind blindness. The
development of this radical proposal as a neurocognitive theory owed much to
the timely coincidence of some highly novel ideas and experiments in the late
1970s and early 1980s. They concerned the need to explain understanding of
mental states, such as beliefs, in the chimpanzee (Premack and
Woodruff, 1978) and in young children (Wimmer and
Perner, 1983). Likewise, there was the need to explain the
spontaneous enjoyment of make believe in infancy (Leslie,
1987). At the same time, it had been documented that young
children with autism lacked spontaneous make-believe play (Wing and
Gould, 1979). New questions could now be asked: how did
understanding of mental states such as belief and pretense evolve? How does
it develop in the normal child? What is different in the brain of individuals
with autism that impairs this development? The Development of Mentalizing in the If
there is a dedicated mechanism for mentalizing, incorporating such functions
as a “decoupler” and an “expression raiser,” when
does it come into play and how does it enable learning? Clearly, a newborn
child does not possess fully functioning mentalizing ability. Nevertheless,
the assumption is that the brain comes equipped with a start-up kit, and that
a normal species-specific social environment will tune it up and get it into
action. The main purpose of an innate start-up mechanism is that it should
lead to fast learning about the properties of its domain, with culture
shaping the content of the knowledge that is acquired. The development of the
social brain involves many other processes as well, such as the perception of
faces, voices, and movements of conspecifics, and these may well be
prerequisites for the development of mentalizing. Sensitivity
and learning about the inner states of agents starts early and proceeds
rapidly. Early signs of such sensitivity are seen in the phenomenon of shared
attention (Carpenter
et al., 1998). Children in the first year of life automatically
follow another person's gaze, seemingly attending to the other person's focus
of interest. Shared attention is accompanied by other signs of mentalizing.
For instance, referential looking, where children check the mother's
expressive attitude toward a novel object before approaching or avoiding it (Repacholi,
1998). The ability to imitate complex and arbitrary but
intentional actions of others—as opposed to their accidental
actions—is another sign of the inexorable progress of mentalizing
ability and is achieved in the middle of the second year of life (Meltzoff,
1995). Young
children aged 2–3 years learn to understand and use mental state verbs
(want, know, pretend) before they learn color names (Bretherton,
1992). Mentalizing ability is also important as a facilitator of
learning in other domains. For instance, according to Bloom
(2000), mentalizing has a critical function in enabling children
to learn the meanings of words. Thus, children don't learn words by mere
association of word sound and object in view. Such association is inherently
ambiguous and error prone, as speaker and listener may look at different
objects. Instead, children learn by tracking the speaker's referential intention,
for example, by taking into account the speaker's gaze (Baldwin et
al. 1996). The effortless ease with which children as young as 5
(and usually before 8 years of age) acquire advanced concepts such as false
belief, deception, white lie, and double bluff is remarkable.
Experimental Studies of Mentalizing Failure in
Autism The
mind blindness theory predicts that the milestones of the normal development
of mentalizing should be absent at the appropriate age in young children with
autism. In particular, they should fail to follow another person's gaze, fail
to point at or show objects of interest—both signs of shared
attention—and fail to understand make-believe play. Baron-Cohen
et al. (1996) looked for these signs in a large prospective
population-based study of infants aged 18 months. At age 3 years, when a firm
diagnosis of autism can be made, the co-occurrence of these early signs was
found to predict the diagnosis remarkably well. Taken together, these three
signs of impaired mentalizing in early life proved reliable enough to serve
as a first infant screening test for autism (Baird et
al., 2000). It is possible that even some preconditions for the
development of mentalizing may be absent. Attentional preferences for human
agents, their faces, their voices, and their movements, which are probably
important triggers for the mentalizing mechanism, may be lacking in autism.
For instance, preschool children with autism did not show a preference for
speech over nonspeech stimuli as do other children (Klin, 1991).
Nor do older children show a spontaneous preference for facial expressions
over other salient stimuli, such as hats (Hobson,
1993). Face recognition difficulties are common throughout the
autism spectrum, perhaps because of a lack of social interest early in life.
In a neuroimaging study, Schultz et
al. (2000) found that brain activation patterns in adults with
autism did not distinguish between faces and objects, in contrast to normal
adults. The
mind blindness hypothesis was originally proposed and tested by Baron-Cohen
et al. 1985 and Baron-Cohen
et al. 1986. The argument was that if the social impairment in
autism arises from a failure of the mentalizing mechanism as conceptualized
by Leslie
(1987), then children with autism should be unable to represent
mental states such as beliefs. They should be unable to understand and
predict behavior in terms of someone's belief even when having achieved the
appropriate level of verbal and cognitive development. The test was a false
belief task originally devised by Wimmer and
Perner (1983), who showed that normally developing children aged 4
and above passed this test. In
the Sally-Ann task, shown in Figure 1,
the following scenario is enacted either with two dolls or two real people:
Sally has a basket and Anne has a box. Sally puts a marble into her basket,
and then she goes out for a walk. While she is outside, naughty Anne takes
the marble from the basket and puts it into her own box. Now Sally comes back
from her walk and wants to play with her marble. Where will she look for the
marble? The answer seems obvious to a 4 year old child: Sally will look
inside her basket. Why? Because that is where Sally thinks it is. The
marble is really in Anne's box, but Sally doesn't know this. She was not
there when Anne transferred the marble. Children with autism, with a mental
age of 4 years and above, had difficulty with this task. Unlike normally
developing children, and unlike children with Down syndrome, they indicated
that Sally would look in Anne's box.
Figure 1. Scenario of Sally-Ann Task With kind
permission from the artist, Axel Scheffler. The
inability of children with autism to understand false belief tasks at the appropriate
age has been confirmed subsequently in a number of studies (see chapters in Baron-Cohen
et al, 1999). Happé
(1995) showed that the verbal mental age of children with autism
who are on the cusp of understanding false belief exceeds that of normally
developing children by 4 or more years. Thus, as shown in Figure 2,
a verbal mental age of 8 and above appears to be necessary to pass standard
false belief tests in the case of autism, but only about 4 in the case of
normal development. Figure
2. Relationships Between Verbal Ability and Success on False Belief Tasks
Redrawn from Happé's meta-analysis of data from 70 children with
autism, 34 children with mental retardation, and 70 normally developing
children, showing the cumulative likelihood of passing false belief tasks
with increasing verbal mental age. With kind permission from the author and
the publishers of Child Development (Blackwell). Implications of Success and Failure on False
Belief Tasks False
belief tasks are deceptively simple, but they tap many different abilities
and can be solved in different ways. The mind blindness hypothesis is often
misunderstood as meaning that people with autism do not possess an explicit
theory of mind and never can possess such a theory. Instead, the hypothesis
is about the failure of the mentalizing start-up mechanism, not about a
“theory.” Despite a dysfunctional start-up mechanism, able
individuals with autism, and especially those with Asperger syndrome, can
come to understand mental states through compensatory learning. However, not
only do they acquire this understanding late, but they are slow and error
prone on more advanced mentalizing tasks. If
success on false belief tasks is not always easy to interpret, neither is
failure. The mentalizing deficit hypothesis predicts failure on the
Sally-Anne and similar tasks, but there are many other reasons for failure.
For instance, the Sally-Anne test requires working memory and the ability to
inhibit reality-oriented responses, i.e., pointing to the place where the
object really is. For a convincing demonstration, it is necessary to show
success on a task that is in every respect the same but that does not involve
thinking about mental states. An
example of such a task uses a scenario in which the critical question
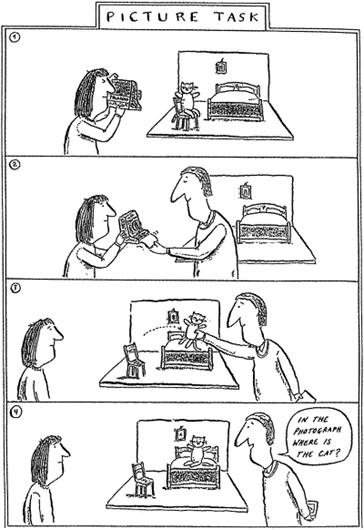
concerned the pictorial content of a photograph. As illustrated in Figure 3,
children were shown a teddy bear sitting on a chair. A Polaroid photograph
was taken of the scene. The photo was put aside, and the teddy bear was moved
to a bed. The critical question was whether the invisible photo showed the
teddy bear on the bed or on the chair. The answer is obviously, “on the
chair.” Compare this to the scenario in the Sally-Anne task, where the
question was whether the invisible belief in Sally's mind was that the marble
was in the basket or in the box. A belief can become false, while still being
held as true in the person's mind. Just so, a photograph can become out of
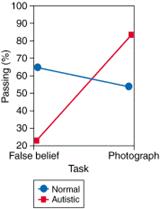
date, still depicting an old scene. The results of a comparison of the two
experiments by Leslie and
Thaiss (1992) are shown in Figure 4.
As predicted by the theory, understanding of false photographs, but not
understanding of false beliefs, was well within the comprehension of children
with autism. In the case of the normally developing children, the situation
was if anything the reverse: they found it easier to answer questions about
Sally's belief than questions about the photograph. This suggests that the
Sally-Ann task requires a measure of inhibitory capacity, and that normally
developing children under the age of 4 struggle with the task because of
domain-general limitations (e.g., inhibitory failure, salience of reality),
while children with autism fail due to specific difficulties in
“reading minds.” This experiment strongly supports the idea that
mentalizing rests on a separable, or modular, cognitive mechanism.
Figure 3. Scenario of False Photograph Task With
kind permission from the artist, Axel Scheffler.
Figure
4. Success and Failure of False Belief and False Photograph Tasks Redrawn
from the results of an experiment by Leslie and Thaiss of 20 normally developing
children with a mean age of 4 years and 15 children with autism with a mean
age of 12 years. Here passing two false belief tasks, one of them illustrated
in Figure 1,
was compared with passing two false photograph tasks, one of them illustrated
in Figure 3.With
kind permission from the authors and the publishers of Cognition (Elsevier).
Mind Blindness Explains the Social Communication
Impairments in Autism The
mind blindness hypothesis is an example of a particular model of
developmental disorders. The claim of this model is that a single
circumscribed cognitive deficit can result in a variety of symptoms that may
superficially look unrelated and that span a wide range of severity (Frith et
al., 1991). Thus, a deficit in mentalizing can account
parsimoniously for the core impairments in socialization, communication, and
imagination that characterize the autism spectrum. At the same time, it is
specific enough to predict unimpaired function in other domains, assuming
there were no additional cognitive deficits. In fact, there are other
deficits. The
mind blindness hypothesis has never claimed to account for the presence of
repetitive behavior and narrow obsessively pursued interests in autism. It
cannot account for motor problems, perceptual processing anomalies, or the
commonly found superior rote memory skills. Other theories address these
features (Russell
1998 and Happé
1999). However, mind blindness may be able to explain some of the
language abnormalities. In autism, muteness, language delay, echoing of
speech, and idiosyncratic use of language are highly typical features. Even
in cases of age-appropriate or precocious appearance of language, a defining
feature of Asperger disorder, parental observations suggest that the first
words were often unusual and that vocabulary acquisition was different from
that in normally developing children. To investigate the apparently odd
pattern of word learning Baron-Cohen
et al. (1997) used the ingenious discrepant looking paradigm,
where speaker and listener attend to different objects while the speaker
utters a new word. They demonstrated that children with autism made errors in
mapping the word to the object that they happened to be looking at at the time,
showing mere association learning. Control children matched for mental age
did not make such errors, but instead mapped the word to the object that the
speaker was looking at. To be guided by the speaker's referential intention
is a sign of mentalizing, and its absence in autism goes some way toward
explaining the unusual development of language in autism. What
of those individuals without the benefit of a start-up mechanism, who learn
about mental states through conscious effort? Slow learning based on forming
associations between behavior and outcomes will allow the gradual acquisition
of mental state concepts. In everyday life, many individuals with autistic
disorder show that they have learned the rules of social convention, but they
lack the intuition to discern situations where these rules become
inappropriate and are thrown by playfulness and irony. However, even without
the intuitive ability to mentalize, social interaction with others who can
make the appropriate allowances for mind blindness can still be a rich source
of experience and learning. Alternative
views of the social impairment in autism have often focused on emotional
dysfunction. Studies by Hobson
(1993) and Sigman and colleagues (see Sigman and
Capps, 1997) suggest that children with autism are less responsive
to the emotions displayed by others. For instance, they show little concern
when an adult cries out in pain, pretending to be hurt, except when their
attention was strongly engaged. On the other hand, contrary to popular
belief, failure of bonding or attachment does not appear to be a
distinguishing characteristic of autism in early childhood. Attachment would
appear to be one of those components of social cognition that are dissociable
from mentalizing. It is possible that responsiveness to specific emotions is
another dissociable social component. Impaired
emotional processing may be secondary to mind blindness. Experimental studies
(Baron-Cohen
et al. 1997 and Adolphs et
al. 2001) suggest that individuals with autism are impaired when
having to interpret complex social emotions from faces rather than simple
basic emotions. Individuals on the severe extreme of the autism spectrum may never
make deliberate eye contact and perhaps may not distinguish between
biological agents and mechanical objects. This severe form of the condition
is characterized by a degree of social detachment that exists over and above
mind blindness. However, global asocial behavior is not the rule in autism
spectrum disorders. Functional Brain Imaging and the Neural Substrate
of Mentalizing As
yet, only a few studies have investigated the neurophysiological substrate of
mentalizing, This is partly due to the difficulties in designing suitable
mentalizing tasks with closely matched control tasks (i.e., tasks that differ
only in the requirement to mentalize). Existing studies of normal volunteers
have used contrasting conditions with stories (Fletcher et
al. 1995; Gallagher
et al. 2000 and Vogeley et
al. 2001), cartoons (Gallagher
et al., 2000), picture sequences (Brunet et
al., 2000), and animated geometric shapes (Castelli et
al., 2000). In all these studies, a network of brain regions was
identified that was consistently active during mentalizing over and above the
specific demands of the respective tasks. This (essentially bilateral)
network appears to be the distinctive signature of mentalizing. The peaks of
activation are in (1) the medial prefrontal cortex, in particular, the most
anterior part of paracingulate cortex, a region on the border between
anterior cingulate and medial prefrontal cortex (very medial); (2) the
temporal-parietal junction at the top of the superior temporal gyrus (stronger
on the right); and (3) the temporal poles adjacent to the amygdala (somewhat
stronger on the left). Figure 5,
Figure 6
and Figure 7
show the location of the average peak activations in the six studies quoted
above. The figures also show peak activations in these same regions obtained
in other highly relevant imaging studies, which can inform us about the
function of these regions and how they might contribute to the ability to
mentalize.
Figure
5. Schematic Representation of Areas in the Medial Prefrontal Cortex
Activated by Mentalizing and Related TasksAverage peak activations during
mentalizing are represented by letters for the following studies: a, Goel et
al., (1995); b, Fletcher et
al., (1995); c, Brunet et
al., (2000); d, Vogeley et
al., (2001); e, Gallagher
et al., (2000); and f, Castelli et
al., (2000). These peaks, which are very medial, cluster in the
most anterior part of the of paracingulate cortex, a region on the border
between anterior cingulate and medial prefrontal cortex, with a range of x
values from −6 to +2 on average (for y and z values, see figure). The
brain volume indicated by the peak activations is circumscribed by a space of
8 × 18 × 24 mm. The same region was also activated in tasks that
required subjects to report on their own mental states as indicated by the
tips of arrows. The self-reported inner states have included actions (Carter et
al., 1998), pain (Rainville
et al., 1997), thoughts (McGuire et
al., 1996), emotions aroused by pictures (Lane et
al., 1997), and sensations experienced during tickling (Blakemore
et al., 1998).
Figure
6. Schematic Representation of Areas in the Temporo-Parietal Region Activated
by Mentalizing and Related Tasks The average peaks of activation in
mentalizing studies are indicated by letters a–f (for key see legend to
Figure 5).
The activations are bilateral, with x values ranging from 41 to 59, but most
studies find greater activations in the right hemisphere, as illustrated in
the diagram. Arrows point to the average peak activation of two studies
involving eye gaze (Wicker et
al. 1998 and Hoffman and
Haxby 2000) and to the average peak activation obtained in four
studies of biological motion (Puce et al.
1998; Bonda et
al. 1996; Calvert et
al. 1997 and Grezes et
al. 1999). A third thicker arrow refers to the area V5/MT which
processes pure motion. This region is activated by all kinds of motion,
whether or not it is biological.
Figure
7. Schematic Representation of Areas in the Peri-Amygdaloid Cortex at the
Temporal Poles Activated by Mentalizing Tasks The average peaks of activation
in mentalizing studies are indicated by letters a–f (for key see legend
to Figure 5).
The activations are bilateral, with x values ranging from 36 to 58, but most
studies find greater activations in the left hemisphere, as illustrated in
the diagram. The peak activation for mentalizing obtained by Baron Cohen
et al. (1999), using an eye gaze interpretation paradigm, is in
the amygdala, which is not represented in this figure as it lies in a region
some 20 mm more medial. Why
these particular regions and what do they have in common? Clearly, the system
identified is tailor-made for processing the intentions of biological agents.
As Figure 5
shows, the same space of the medial frontal region is also activated by tasks
that imply awareness of the self. As Figure 6
shows, the superior temporal sulcus, mainly on the right, is also activated
by tasks that require detection of biological agents. Further studies suggest
that this is not confined to biological motion in the visual modality.
Activations are shown with such diverse stimuli as faces (Kesler-West
et al., 2001), speech (Belin et
al., 2000), multimodal cues (Kawashima
et al., 1999), and contextual cues to intention (Toni et
al., 2001). Other studies suggest that the temporal poles,
stronger on the left, are also activated when facts about other agents and
the self are remembered, e.g., familiar faces and scenes (Nakamura et
al., 2000), and familiar emotionally laden stimuli in different
modalities (Dolan et
al., 2000). Unfortunately,
none of the studies to date throws light on how a link between all three
regions might result in mentalizing. This ability is clearly more than the
sum of its parts. If mentalizing crucially involves “decoupling”
to keep apart real states of affairs and mental states (Leslie,
1987), then what neurophysiological process might underpin it? One
key approach to this problem is the comparative study of brain physiology in
autism. If mentalizing is dysfunctional in autism, then the associated brain
abnormality should point us in the right direction. Evidence from Functional Brain Imaging of
Mentalizing in Autism Only
three studies to date have explicitly studied individuals with autism on
mentalizing tasks. Happé et al. used a story paradigm in a PET study,
comparing six normal adults with five able adults with Asperger syndrome.
Subjects were scanned while reading stories and answering questions about
complex mental states or nonmental inferences, against a baseline of reading
and remembering unconnected sentences. While both types of subjects answered
the questions satisfactorily, differences were shown in brain activation. The
Asperger group showed less activation in the critical medial prefrontal
region, while their peak activation was in a more ventral region of frontal
cortex. In
an fMRI study, Baron-Cohen
et al. (1999) compared a group of six able individuals with autism
with a group of twelve controls. Subjects were asked to judge inner states of
people from photographs of the eye region, deciding which of two words best
described their mental/emotional state. The contrast was to judge whether the
photo was that of a male or a female. Compared to the control group, people
with autism demonstrated less extensive activation in frontal regions and no
activation in the amygdala. Castelli
et al. (unpublished data) showed silent animations to ten able adults of
normal intelligence with autistic disorder and to ten normal adults. The
animations featured two triangles moving about on a screen [for examples see http://www.icn.ucl.ac.uk/groups/UF/Research/animations.html].
In one condition they were scripted to elicit attribution of mental states
(e.g., coaxing, mocking). In another condition, the triangles moved randomly.
This was the contrast that was used to highlight the mentalizing system.
During mentalizing, the autism group showed less activation than the controls
in the three previously identified brain regions. However, they showed
identical activation during mentalizing in one additional region, the
occipital gyrus. The activation of this region suggests that both groups
devoted more intensive visual analysis to the critical animations. However,
connectivity between the occipital and temporo-parietal regions was weaker in
the autism group than in the controls. This finding provides a clue to a
possible reason for mind blindness. The underactivation of the system may be
due to a bottleneck for interactive influences between lower and higher order
perceptual processing areas. These findings are still preliminary but support
the notion of a dysfunction in the specific neural substrate for mentalizing
in autism. Evidence from Anatomical Studies of the Brain in
Autism Can
mentalizing failure in autism be linked to some structural abnormality in one
or more of the regions of the mentalizing system? Some preliminary evidence
for such a possibility exists. Abell et
al. (1999) reported structural magnetic resonance imaging (MRI)
data on 15 high-functioning individuals with autistic disorder. A voxel-based
whole brain analysis identified gray matter differences relative to 15 age-
and IQ-matched controls in a distributed system possibly centered on the
amygdala. Decreases of gray matter were found in anterior parts
of this system, in particular the paracingulate sulcus and inferior frontal
gyrus. The paracingulate region was extremely close to the region that was
found to be less active in individuals with autistic disorder in the Happé
et al. (1996) and Castelli et al. (unpublished data) imaging
studies. Increases in gray matter were also found in the posterior
parts, that is, the peri-amygdaloid cortex and the middle temporal and
inferior temporal gyrus. Increases in cerebellar structures were also found.
Another structural MRI study (Howard et
al. 2000), using volumetric measures, also found an enlargement in
the amygdaloid region in able individuals with autistic disorder. While there
are theories of amygdala dysfunction in autism (Baron-Cohen
et al. 2000; Howard et
al. 2000 and Adolphs et
al. 2001), the evidence so far suggests that this region is only
one component among several that might play a causal role in the origin of
mind blindness. There
is also evidence from the few existing histoanatomical studies of autistic
brains for abnormalities in these particular brain regions. For example, Bauman and
Kemper (1994), in an important series of studies, reported
cellular abnormalities in post mortem brains of individuals with autistic
disorder, in particular, reduced neuronal cell size and increased cell
packing density in regions of the limbic system comprising the hippocampal
complex, subiculum, entorhinal cortex, amygdala, mamillary body, medial
septal nucleus, and anterior cingulate. Outside the limbic system, reduced
numbers of Purkinje cells were found in the posterior and inferior regions of
the cerebellum. Evidence from Acquired Brain Lesions Given
that the anterior part of paracingulate cortex, the superior temporal sulcus
at the temporo-parietal junction, and the temporal poles, have been reliably
activated in neuroimaging studies of mentalizing, what can we learn from
acquired lesions of these areas? We do not expect to find patients suffering
the equivalent of autism. For one thing, the effects of developmental brain
abnormalities would be different from those of accidentally acquired lesions;
for another, there is more to autism than social communication impairment.
However, we can gain information on whether intact functioning of these
regions is necessary for mentalizing success. Some
studies exist where typical theory of mind tasks have been used with the
appropriate control tasks in patients with brain lesions. Happé
et al. (2001) showed that a patient who had undergone stereotactic
anterior capsulotomy (which severs fronto-thalamic fibers) for intractable
depression was specifically impaired on mentalizing tasks following surgery.
He was reported to show deterioration in his everyday social behavior. He
also failed cartoon tests and story tests of theory of mind. Group studies of
patients with prefrontal lesions, which most probably included the critical
medial prefrontal region identified in brain imaging studies, also show
theory of mind deficits on a variety of tasks (Stone et
al. 1998; Channon and
Crawford 2000 and Stuss et
al. 2001). Importantly, the evidence from the patients who suffer
mentalizing failure suggests independence from performance on executive
function tasks, which is also thought to be dependent on frontal lobe function
(Rowe et al.
2001 and Blair and
Cipolotti 2000). Reports
on patients with damage to the superior temporal sulcus at the
temporo-parietal junction, mainly on the right, have not so far included
mentalizing tasks. However, the right hemisphere stroke patients studied by Happé
et al. (1999) with verbal and nonverbal mentalizing tasks could
well have included such lesions. These authors found impairments and
communication failure as typically seen in some cases of autism, but only in
their right hemisphere patients, not in their left hemisphere patients. A
study of a patient with congenital left amygdaloid lesion and a diagnosis of
Asperger syndrome showed severe impairment on large variety of mentalizing
tasks (Fine et
al., 2001). It would be interesting to study mentalizing
performance in patients with semantic dementia who suffer from lesions in the
temporal pole. The
neuropsychological studies to date suggest that the medial prefrontal cortex
may be necessary for mentalizing, but it seems unlikely that it is also
sufficient. For lesions in other regions identified as part of the
mentalizing system in brain imaging studies, data are as yet too sparse. Other
lesion cases too could be informative, in particular in the cerebellum, which
has been found to be active during mentalizing in at least some of the few
extant studies. In
summary, the results from neuropsychological, structural, and functional
imaging studies to date, together with findings on cellular abnormalities in
autistic brains, provide some converging evidence for the critical brain
abnormalities leading to mind blindness. Preliminary Thoughts on the Evolution of Mind
Reading The
social brain is complex (Brothers,
1997), and very old, but the mentalizing system appears to be of
more recent origin. Monkeys, who are known for their complex social lives,
are unable to mentalize (Cheney and
Seyfarth, 1990), in contrast to chimpanzees and bonobos, who
appear to have only incipient mentalizing skills but can engage in deception (de Waal,
1992). Mentalizing adds a new dimension to the repertoire of
social interactions. It allows the manipulation of others in particularly
subtle ways and reaches far beyond the ability to manipulate their behavior
by direct instrumental action. Frith and
Frith (2000) speculated that the brain system dedicated to the
representation of mental states evolved from the dorsal action system rather
than from the ventral object identification system. They argued that much of
the social intelligence already so well developed in the monkey could be seen
as deriving from the ventral system. It depends upon complex and
sophisticated object recognition: recognition of subtle differences in
emotional expression, recognition of other individuals, and recognition of
their status and relationships. Mentalizing, in contrast, required the
development of the capacity to represent actions, and the goals and
intentions of agents implicit in actions performed by agents. Both
goal directed movement and eye gaze of other agents provide clues to their
desires, and the ability to detect such clues may be a first step in the
evolution necessary for mentalizing. The ability to detect goal directed
movements is already found in animals without even the incipient ability to
mentalize. Neuroimaging studies have pinpointed the temporal-parietal
junction at the top of the superior temporal sulcus during both the detection
of eye gaze and of mentalizing (see Figure 6).
What is known about cells in this part of the cortex? In their work with
monkeys, Perrett et
al. (1989) have identified cells in the superior temporal sulcus
(STS) that respond to moving hands and faces but not to the movement of
inanimate objects. Moreover, cells in STS, just as the “mirror
neurons” (Gallese et
al. 1996) in lateral inferior frontal regions of the macaque brain
(F5), respond to the observation of specific actions (e.g., a precision
grip). Intriguingly, a neuron in anterior cingulate cortex (close to the area
with peak activations in mentalizing studies [Figure 5])
in a patient undergoing neurosurgery was found to respond when the patient
received a pinprick and also when he watched pinpricks to the examiner's
fingers (Hutchison
et al. 1999). It is plausible that mirror mechanisms form an early
evolutionary link to mentalizing. Speculatively, their function underpins not
only the automatic computation of an agent's goal directed actions, but of an
agent's intention toward the self (prey or predator; friend or foe). However,
the detection of agency still does not get us anywhere near the ability to
mentalize. How and where might this task be accomplished by neurons? The
medial frontal cortex, in particular the most anterior part of the
paracingulate cortex, is a promising candidate for the critical next step
toward the evolution of mentalizing. First, it is active during the
attribution of mental states to others and during the monitoring of inner
states of the self (see Figure 5).
Second, lesions in this area have been associated with mentalizing failure.
Third, abnormal function as well as abnormal structure has been shown in
autistic individuals in this region. Very
little is known about cells in anterior cingulate and adjacent medial
prefrontal areas. However, an unusual type of projection neuron, spindle
cells, has been identified in the anterior cingulate cortex (layer Vb) of
bonobo, chimpanzee and man, but not in any other primate species or other
mammals (Nimchinsky
et al. 1999). The authors suggest that spindle cells in the
anterior cingulate might represent a population of specialized neurons that
could integrate inputs with emotional overtones and project to motor centers
controlling vocalization or facial expression. While the function of the
spindle cells is as yet unknown, it is notable that their appearance
coincides with observations of incipient mentalizing in chimpanzees and
bonobo but lack of mentalizing in monkeys. Concluding Remarks Mind
blindness makes sense of the core social and communication impairments of
individuals with autism. The hypothesis rests on robust experimental evidence
and has the unique advantage of unifying the core symptoms that define the
spectrum of autistic disorders by a single explanation and is able to account
for the heterogeneity that is associated with autistic spectrum disorders. Mentalizing
rests on a separable brain system and can be selectively damaged by acquired
brain lesions. The physiological basis of mentalizing remains unknown and
appears to involve a complex, essentially bilateral network of cortical
regions. In imaging studies, the most consistently activated regions are
paracingulate sulcus in medial prefrontal regions, superior temporal sulcus
at the temporo-parietal junction (more strongly on the right), and
peri-amygdaloid cortex at the temporal poles (more strongly on the left).
Preliminary findings suggest that the brain abnormality that results in
autism compromises the functional connectivity of this network and leads to
reduced activations in all three regions. Converging evidence from autism and
acquired brain lesions suggests that an intact medial prefrontal region is
necessary for mentalizing. Experimental
evidence shows that the typical social communication impairment of autism can
be well explained by impairment in the mentalizing mechanism. Able
individuals with autism spectrum disorders can with time and practice achieve
awareness of mental states by compensatory learning. In normally developing
children, the mentalizing mechanism allows fast learning of socially and
culturally transmitted knowledge, including the meaning of words. Since
children with autism spectrum disorders can be very intelligent and can learn
by other means, the underlying brain abnormality must be sufficiently
specific and circumscribed so as not to compromise general information
processing ability. This has implications for a modular view of the
development of cognitive functions. References Abell,
F., Krams, M., Ashburner, J., Passingham, R., Friston, K., Frackowiak, R.,
Happé, F., Frith, C. and Frith, W., 1999. The neuroanatomy of autism:
a voxel-based whole brain analysis of structural scans. Neuroreport 10,
pp. 1647–1651. Adolphs,
R., Sears, L. and Piven, J., 2001. Abnormal processing of social information
from faces in autism. J. Cog. Neurosci. 13, pp. 232–240. American
Psychiatric Association. (2000). Diagnostic and Statistical Manual of Mental
Disorders, 4th Edition DSM-IV-TR (Text Revision) ( Asperger,
H., 1944. “Autistic psychopathy” in childhood. In: Autism and
Asperger Syndrome, Editor, , 1944. In, Bailey,
A.J., Phillips, W. and Rutter, M., 1996. Autism: integrating clinical,
genetic, neuropsychological, and neurobiological perspectives. J. Child
Psychol. Psychiatry. 37, pp. 89–126. Baird,
G., Charman, T., Baron-Cohen, S., Cox, A., Swettenham, J., Wheelwright, S.
and Drew, A., 2000. A screening instrument for autism at 18 months of age: a
6-year follow-up study. J. Am. Acad. Child Adolesc. Psychiatry. 39,
pp. 694–702. Baldwin,
D.A., Markman, E.M., Bill, B., Desjardins, R.N., Irwin, J.M. and Tidball, G.,
1996. Infants' reliance on a social criterion for establishing word-objects
relations. Child Dev. 67, pp. 3135–3153. Baron-Cohen,
S., 1995. Mindblindness: An Essay on Autism and Theory of Mind, MIT
Press, Baron-Cohen,
S., Leslie, A. and Frith, U., 1985. Does the autistic child have a
“theory of mind”?. Cognition 21, pp. 37–46. Baron-Cohen,
S., Leslie, A. and Frith, U., 1986. Mechanical, behavioural and intentional
understanding of picture stories in autistic children. Brit. J. Dev.
Psych. 4, pp. 113–125. Baron-Cohen,
S., Cox, A., Baird, G., Swettenham, J., Nightingale, N., Morgan, K., Drew, A.
and Charman, T., 1996. Psychological markers in the detection of autism in
infancy in a large population. Brit. J. Psychiatry 168, pp.
158–163. Baron-Cohen,
S., Wheelwright, S. and Joliffe, T., 1997. Is there a “language of the
eyes”? Evidence from normal adults and adults with autism or Asperger
syndrome. Visual Cognition 68, pp. 48–57. Baron-Cohen,
S., Tager-Flusberg, H. and Cohen, D., 1999. Understanding Other Minds II:
Perspectives from Autism and Cognitive Neuroscience, Baron-Cohen,
S., Ring, H.A., Bullmore, E.T., Wheelwright, S., Ashwin, C. and Williams, S.C.R.,
2000. The amygdala theory of autism. Neurosci. Biobehav. Rev. 24,
pp. 355–364. Bauman,
M. and Kemper, T.L., 1994. Neuroanatomic observations of the brain in autism.
In: Bauman, M. and Kemper, T.L., Editors, 1994. The Neurobiology of Autism,
Belin,
P., Zatorre, R.J., Lafaille, P., Ahad, P. and Pike, B., 2000. Voice-selective
areas in human auditory cortex. Nature 403, pp. 309–312. Black,
Blair,
R.J.R. and Cipolotti, L., 2000. Impaired social response reversal: A case of
“acquired sociopathy.”. Brain 123, pp.
1122–1141. Blakemore,
S.J., Wolpert, D. and Frith, C.D., 1998. Central cancellation of
self-produced tickle sensation. Nat. Neurosci. 1, pp.
635–640. Bloom,
P., 2000. How Children Learn the Meaning of Words, MIT Press, Bonda,
E., Petrides, M., Ostry, D. and Evans, A., 1996. Specific involvement of
human parietal systems and the amygdala in the perception of biological
motion. J. Neurosci. 16, pp. 3737–3744. Bretherton,
Brothers,
L. (1997). Friday's Footprint: How Society Shapes the Human Mind ( Brunet,
E., Sarfate, Y., Hardy-Bayle, M.C. and Decety, J., 2000. A PET investigation
of the attribution of intentions with a nonverbal task. Neuroimage 11,
pp. 157–166. Calvert,
G.A., Bullmore, E.T., Brammer, M.J., Carpenter,
M., Nagell, K. and Tomasello, M., 1998. Social cognition, joint attention and
communicative competence from 9 to 15 months of age. Monogr. Soc. Res.
Child Dev. 63, pp. 1–143. Carter,
C., Braver, T., Barch, D., Botvinick, M., Noll, D. and Cohen, J., 1998.
Anterior cingulate cortex, error detection, and the online monitoring of
performance. Science 280, pp. 747–749. Castelli,
F., Happé, F., Frith, U. and Frith, C.D., 2000. Movement and mind: A
functional imaging study of perception and interpretation of complex
intentional movement patterns. Neuroimage 12, pp.
314–325. Channon,
S. and Crawford, S., 2000. The effects of anterior lesions on performance of
a story comprehension test: left anterior impairment on a theory of mind-type
task. Neuropsychologia 38, pp. 1006–1017. Cheney,
D.L. and Seyfarth, R.M., 1990. How monkeys see the world: Inside the mind
of another species, Chicago University Press, Chicago. Dolan,
R.J., Lane, R., Chua, P. and Fletcher, F., 2000. Dissociable temporal lobe
activations during emotional episodic memory retrieval. Neuroimage 11,
pp. 203–209. Fine,
C., Lumsden, J. and Blair, J., 2001. Dissociation between “theory of
mind” and executive functions in a patient with early left amygdala
damage. Brain 124, pp. 287–298. Fletcher,
P.C., Happé, F., Frith, U., Baker, S.C., Dolan, R.J., Frackowiak,
R.S.J. and Frith, C.D., 1995. Other minds in the brain: A functional imaging
study of “theory of mind” in story comprehension. Cognition
57, pp. 109–128. Fombonne,
E., 1999. The epidemiology of autism: a review. Psychol. Med. 29,
pp. 769–786. Frith,
U. and Happé, F., 1998. Why specific developmental disorders are not
specific: On-line and developmental effect in autism and dyslexia. Dev.
Sci. 1, pp. 267–272. Frith,
C.D. and Frith, U., 2000. The physiological basis of theory of mind. In:
Baron-Cohen, S., Tager-Flusberg, H. and Cohen, D.J., Editors, 2000. Understanding
Other Minds, Perspectives from Developmental Cognitive Neuroscience
(Second Edition ed.), Frith,
U., Leslie, A. and Morton, J., 1991. The cognitive basis of a biological
disorder. Trends Neurosci. 14, pp. 433–438. Gallagher,
H., Happé, F., Gallese,
V., Fadiga, L., Fogassi, L. and Rizzolatti, G., 1996. Action recognition in
the prefrontal cortex. Brain 119, pp. 593–609. Grezes,
J., Costes, N. and Decety, J., 1999. The effects of learning and intention on
the neural network involved in the perception of meaningless actions. Brain
122, pp. 1875–1887. Goel,
V., Grafman, J., Sadato, N. and Hallett, M., 1995. Modelling other minds. Neuroreport
6, pp. 1741–1746. Happé,
F., 1995. The role of age and verbal ability in the theory of mind task
performance of subjects with autism. Child Dev. 66, pp.
643–855. Happé,
F., 1999. Autism: Cognitive deficit or cognitive style. Trends Cog. Sci.
3, pp. 216–222. Happé,
F. and Frith, U., 1996. The neuropsychology of autism. Brain 119,
pp. 1377–1400. Happé,
F., Ehlers, S., Fletcher, S., Frith, U., Johannsson, M., Gillberg, C., Dolan,
R., Frackowiak, R. and Frith, C., 1996. “Theory of mind” in the
brain. Evidence from a PET scan study of Asperger Syndrome. Neuroreport
8, pp. 197–201. Happé,
F., Brownell, H. and Winner, E., 1999. Acquired “theory of mind”
following stroke. Cognition 70, pp. 211–240. Happé,
F., Malhi, G. and Checkley, S., 2001. Acquired mind-blindness following
frontal lobe surgery? A single case study of impaired “theory of
mind” in a patient treated with stereotactic anterior capsulotomy. Neuropsychologia
39, pp. 83–90. Hobson,
P., 1993. Autism and the Development of Mind, Erlbaum Assoc, Hoffman,
E.A. and Haxby, J.V., 2000. Distinct representations of eye gaze and identity
in the distributed human neural system for face perception. Nat. Neurosci.
3, pp. 80–84. Howard,
M., Cowell, P., Boucher, P., Broks, P., Mayes, A., Farrant, A. and Roberts,
N., 2000. Convergent neuroanatomical and behavioural evidence of an amygdala
hypothesis of autism. Neuroreport 11, pp. 2931–2935. Hutchison,
W.D., Davis, K.D., Lozano, A.M., Tasker, R.R. and Dostrovsky, J.O., 1999.
Pain-related neurons in the human cingulate cortex. Nat. Neurosci. 2,
pp. 403–405. Kanner,
L., 1943. Autistic disturbances of affective contact. Nervous Child 2,
pp. 217–250. Kawashima,
R., Imaizumi, S., Mori, K., Okada, K., Goto, R., Kiritani, S., Ogawa, A. and
Fukuda, H., 1999. Selective visual and auditory attention toward utterances
– a PET study. Neuroimage 10, pp. 209–215. Kesler-West,
M.L., Andersen, A.H., Smith, C.D., Avison, M.J., Klin,
A., 1991. Young autistic children's listening preferences in regard to
speech: A possible characterization of the symptom of social withdrawal. J.
Autism Dev. Disord. 21, pp. 29–42. Lane,
R.D., Fink, G.R., Chua, P.M. and Dolan, R.J., 1997. Neural activation during
selective attention to subjective emotional responses. Neuroreport 8,
pp. 3969–3972. Leslie,
A., 1987. Pretence and representation: The origins of “theory of
mind.”. Psychol. Rev. 94, pp. 412–426. Leslie,
A. and Thaiss, L., 1992. Domain specificity in conceptual development:
Evidence from autism. Cognition 43, pp. 467–479. Lord,
C., Cook, E.H., Leventhal, B.L. and Amaral, D.G., 2000. Autism spectrum
disorders. Neuron 28, pp. 355–363. Maestrini,
E., Paul, A., McGuire,
P.K., Silbersweig, D.A. and Frith, C.D., 1996. Functional neuroanatomy of
verbal self-monitoring. Brain 119, pp. 907–917. Meltzoff,
A., 1995. Understanding the intentions of others: Reenactment of intended
acts by 18-month-old children. Dev. Psychol. 31, pp.
838–850. Nakamura,
K., Kawashima, R., Sato, N., Nakamura, A., Sugiura, M., Kato, T., Hatano, K.,
Ito, K., Fukuda, H. et al., 2000. Functional delineation of the human
occipito-temporal areas related to face and scene processing. Brain 123,
pp. 1903–1912. Nimchinsky,
E.A., Gilissen, E., Allman, J.M., Perl, D.P., Erwin, J.M. and Perrett,
D.I., Harries, M.H., Bevan, R., Thomas, S., Benson, P., Mistlin, A., Chitty,
A., Hietanen, J. and Ortega, J., 1989. Frameworks of analysis for the neural
representation of animate objects and actions. J. Exp. Biol. 146,
pp. 87–113. Premack,
D. and Woodruff, G., 1978. Does the chimpanzee have a theory of mind?. Behav.
Brain Sci. 1, pp. 515–526. Puce,
A., Allison, T., Bentin, S., Gore, J.C. and McCarthy, G., 1998. Temporal
cortex activation in humans viewing eye and mouth movements. J. Neurosci.
18, pp. 2188–2199. Rainville,
P., Repacholi,
B.M., 1998. Infants' use of attentional cues to identify the referent of
another person's emotional expression. Dev. Psychol. 34, pp.
1017–1025. Rowe,
A.D., Bullock, P.R., Polkey, C.E. and Morris, R.G., 2001. “Theory of
mind” impairments and their relationship to executive functioning
following frontal lobe excisions. Brain 124, pp. 600–616.
Russell,
J., Editor, , 1998. Autism as an Executive Disorder, Scheuffgen,
K., Happé, F., Schultz,
R.T., Gauthier, I., Klin, A., Fulbright, R.K., Sigman,
M. and Capps, L., 1997. Children with Autism. A Developmental Perspective,
Stone,
V.E., Baron-Cohen, S. and Knight, R.T., 1998. Frontal lobe contributions to
theory of mind. J. Cog. Neurosci. 10, pp. 640–656. Stuss,
D., Toni,
T., Thoenissen, D. and Zilles, K., 2001. Movement preparation and motor
intention. Neuroimage 14, pp. S110–S117. de Waal,
F.B.M., 1992. Intentional deception in primates. Evol. Anthropol. 1,
pp. 86–92. Vogeley,
K., Bussfeld, P., Newen, A., Herrmann, S., Happé, F., Falkai, P.,
Maier, W., Shah, N.J., Fink, G.R. and Zilles, K., 2001. Mind reading: Neural
mechanisms of theory of mind and self-perspective. Neuroimage 14,
pp. 170–181. Wicker,
B., Michel, F., Henaff, M.A. and Decety, J., 1998. Brain regions involved in
the perception of gaze: a PET study. Neuroimage 8, pp.
221–227. Wimmer,
H. and Perner, J., 1983. Beliefs about beliefs: Representation and
constraining function of wrong beliefs in young children's understanding of
deception. Cognition 13, pp. 103–128. Wing,
L. and Gould, J., 1979. Severe impairments of social interaction and
associated abnormalities in children: Epidemiology and classification. J.
Autism Dev. Disord. 9, pp. 11–29. World
Health Organization, 1992. The ICD-10 classification of mental and
behavioural disorders: Clinical Descriptions and Diagnostic Guidelines,
World Health Organization, |