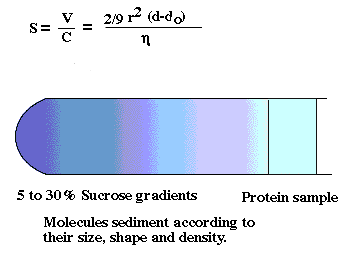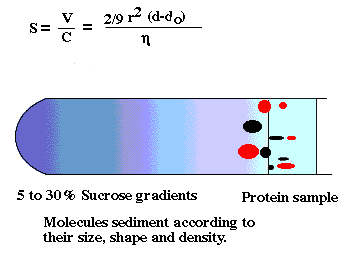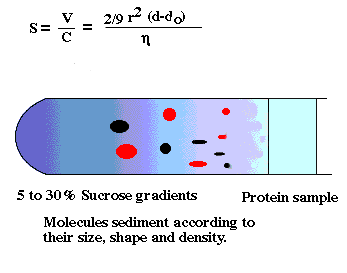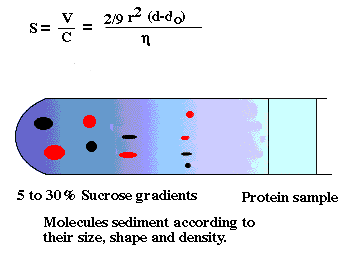
image from http://ntri.tamuk.edu/centrifuge/centrifugation.html
Microscopy
I. Introduction
A. Cell biology is one of the youngest branches of the life sciences
B. Initial Considerations
1. Why is the
observation of biological structure is difficult to accomplish?
2. Resolution and contrast
II. Theoretical Considerations in Microscopy
A. Light Microscopy (LM)
1. What is resolving power?:
2. What does resolving power depend on?
3. How can the limit of resolution (LR) be determined?
LR = 0.61 (lambda)/NA
NA = n x sině (light collecting ability of lens)
NA: the maximum of an oil immersion lens = 1.4 (for a dry lens = 1)
4. What does LR depend on (from Abbes's Equation)?
- bright field microscopes
- phase contrast, Nomarski, darkfield, confocal microscopes, etc.
1. Resolving power compared with LM
- the electron "beam" wavelength
-What determines the wavelength of an electron beam
LR = 0.34 nm (at 100,000 volts, LR = 0.24 nm)
2. How are resolution and magnification related?
3. Some limitations of EM
-low penetration power of electrons
-the tissue must be impregnated with a tough plastic resin and extremely thin
sections cut
-the column of the EM must be a vacuum
-visualization of the specimen requires a specialized screen
Cell and Tissue Fractionation
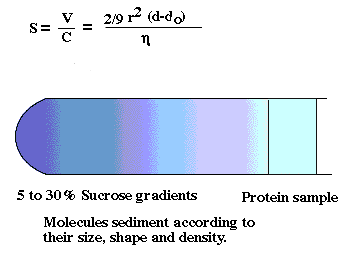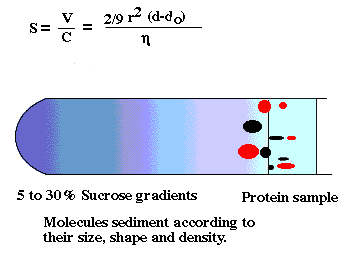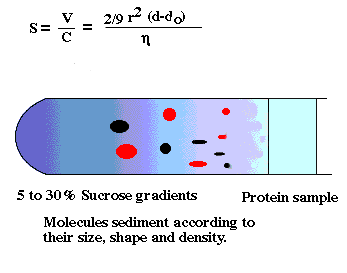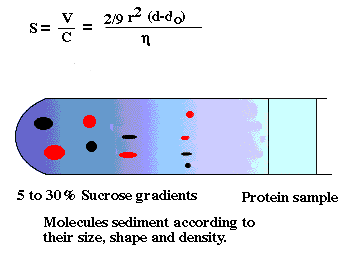
image from http://ntri.tamuk.edu/centrifuge/centrifugation.html
I. Introduction
II. The Three Phases
A. Disruption or homogenization
B. Fractionation
C. Analysis of the cell fractions
A. Homogenization
The goal?
What may
damage cell components?
1. osmotic shock
What happens if organelles are released into distilled water?
What about into a strong salt solution?
How to mimimize osmotic shock
2. activity of degradative enzymes
What happens when you break open a cell?
Under what conditions do lysosomal enzymes work best?
What do you do to minimize damage due to degredative enzymes?
3. shearing force of the homogenizer
What happens when you homogenize a cell?
Examples of methods used:
Overall comment: The homogenization technique chosen depends
on the goal of the homogenization and the tissue used.
Review
What is the homogenate?
What makes up a typical homogenization medium?
B. Fractionation
Review
F = m (omega)2x
where m = mass of the particle; (omega) = angular velocity of spinning rotor
(radians/sec) and x = distance of the particle from the axis of rotation.
For convenience, F is usually measured in terms relative to the gravitational
pull of the earth, so:
RCF = Relative Centrifugal Force
RCF = Fcentrifuge/Fgravity
RCF = 1.119 x 10-5 (rpm)2 (x)
Note that RCF is also often called the "g" force
The "s" values for a number of components have been determined
using analytical ultracentrifugation.
Thus, "s" values are expressed as a unit of time in seconds.
The Svedberg unit (after builder of the analytical centrifuge) is equal to 10-13
seconds; a table of "S" values will show whole
number values for many common cellular constituents.
|
Cell Constituent |
S (x 10-13) |
| myoglobin | 1.8 |
| ribosome | 70 |
| lysosomes | 4000 |
| peroxisomes | 4000 |
| chloroplast | 100,000 |
The "S" coefficients are the theoretical basis for centrifugation
techniques which separate cell components based upon differences in sedimentation rate.
The type of centrifugation which is used to separate cell components is called preparative
centrifugation.
Two basic procedures:
-differential centrifugation
-density gradient centrifugation
differential centrifugation
-contamination
-wall effects
density gradient centrifugation
-cell fractions obtained with this method tend to be cleaner than
differential pellets. Why?
-Density values of many organelles also overlap
-Wall effects in a swinging bucket rotor are less than a fixed angle rotor.
