In a postulated
mechanism, Glu98 activates a water molecule, which attacks the phosphorus
atom on the 1-phosphate of fructose 1,6-bisphosphate (figure 8). Asp121
and the C2-hydroxyl group may assist the transfer of a proton to the bridging
oxygen (8). The hydrolysis of a phosphate ester can proceed through an
intermediate of metaphosphate (dissociative mechanism) or through a trigonal
bipryamidal transition state (associative mechanism). Model systems in
solution support the dissociative pathway, whereas most enzymologists favor
an associative mechanism for enzyme-catalyzed reactions (7). It is difficult
to specify a mechanism that would work for all F1,6-BPase activity in living
organisms.
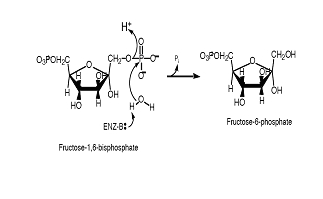 |
| Figure 8. Mechanism for F 1,6-BPase
catalysis. The enzyme activates a water molecule, which attacks the 1-Phosphate.
The bridging oxygen becomes protonated and fructose 6-phosphate is produced
along with a Pi molecule. California
Institute of Technology. |
|
|