Procedural Notes
- The styrofoam cups are actually better than the aluminum calorimeters for this.
- Weigh the warm water rather than just record the volume from the beaker. The beaker is probably precise enough, but you have to weigh the whole thing afterwards to determine the mass of the ice, so you may as well just be consistent.
- Only use a small amount of ice. About 30g by weight is about what fits in the smallest beaker. Add the ice to calorimeter carefully, without pouring in any of the meltwater.
- The ice melts very rapidly. Depending on the warm water temperature, it takes only about 2-3 minutes. You can stop data collection if all the ice has melted and the temperature has stopped dropping.
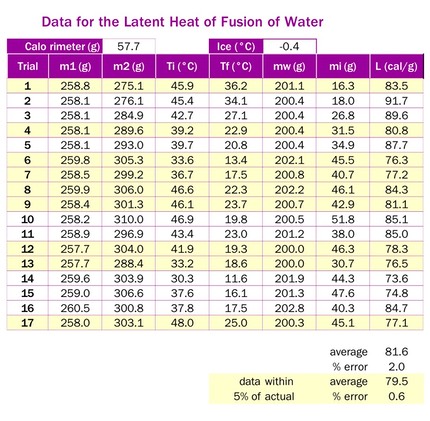
- Multiple trials are very desirable here. There seems to be a lot of inconsistency in the results. After 17 trials, only 9 were within 5% of the known value of the latent heat. There were outliers on both sides, though, and the average of all the data had a 1.6% error. The subset of the data which was within 5% of the known value had an average of 79.5 cal/g, or a 0.6% error.